Background: Median age at diagnosis of diffuse large B cell lymphoma (DLBCL) patients is 66 years; 40% of patients are diagnosed at an age greater than 70 years. CAR-T cell therapy is approved for the treatment of relapsed/refractory DLBCL patients; however, a deeper understanding of outcomes in patients older than 70 years is needed. Our aim was to analyze outcomes of CAR-T cell therapy in this population and compare them to those obtained in younger patients in the real-world setting.
Methods: A subgroup analysis of our real life experience report (Kwon, Iacoboni et al. Haematologica 2022) was performed. Data from consecutive patients treated in Spain with commercial CAR-T products were retrospectively collected on behalf of GETH-TC (Spanish Group of Stem Transplantation and Cell Therapy)-GELTAMO (Spanish Group of Lymphoma and Autologous Stem Cell Transplantation). Patients infused between November-2018 and August-2021 were included. Last update of the cohort was performed in June 2023. Cytokine release syndrome (CRS) and Immune effector cell-associated neurotoxicity syndrome (ICANS) were graded using the ASTCT consensus criteria. Response was assessed according to the Lugano criteria.
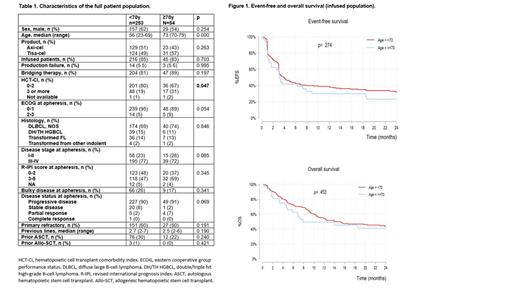
Results: Characteristics of the patients are summarized in Table 1. A total of 307 patients underwent apheresis for CAR-T cell therapy as 3 rd or subsequent line of therapy. Fifty-four (18%) patients were 70 years or older. There were no differences between groups regarding product selection, proportion of patients infused, production failure and rate of bridging therapy. There were more patients with HCT-CI score ≥3 and a trend of higher proportion of patients with ECOG >2 at apheresis in the older group (31% vs. 19%, p=0.047; 9% vs. 5%, p=0.054); the remaining baseline characteristics did not differ. A similar proportion of patients were infused.
Among the infused population (n=261), median time from apheresis to infusion was 49 days for patients ≥70 years (n=45) and 47 for younger patients (n=216) (p=0.824). A similar proportion of patients developed any grade of CRS and ICANS (88% vs. 80%, p=0.132 and 31% vs. 29%, p=0.843) and grade 3-4 events (11% vs. 6.5%, p=0.277 and 18% vs. 10%, p=0.146). Median duration of CRS (4 and 5 days, p=0.150), ICANS (4 and 5 days, p=0.540) and hospitalization length (20 days for both groups, p=0.995) did not differ between groups. There were not significant differences in the proportion of patients admitted to ICU (24% vs. 18%, p=0.284); however, the proportion of patients developing infection in the first 6 months after infusion showed a trend to be higher in the older group (42% vs. 29%, p=0.099). Non-relapse mortality was similar between groups (13% vs. 7%, 9=0.24). With a median follow-up of 19.2 months from infusion, both 12-m PFS (30% vs. 38%, p=0.281) and 12-m OS (49% vs. 54%, p= 0.473) were similar between older and younger patients (Figure 1).
In the multivariate analysis of the whole population including CAR-T type, disease characteristics (primary refractory, progressive disease, disease stage, R-IPI and high LDH at apheresis), ECOG ≥2 and age ≥70, high LDH (HR 2.1, p=0.001), disease stage III-IV (HR 1.8, p=0.038) and ECOG ≥2 (HR 2, p=0.025) were risk factors for EFS. Age ≥70 (HR 1.5, p=0.054) and progressive disease at lymphodepletion (HR 1.8, p=0.055) were nearly significant risk factors. In the multivariate analysis for OS, high LDH (HR 1.7, p=0.013), high R-IPI (1.2, p=0.039), ECOG ≥2 (HR 2, p=0.014) and progressive disease at lymphodepletion (HR 1.9, p0.042) were risk factors for OS, while age had no impact (HR 1.1, p=0.674).
Conclusions: In our real-life experience, CAR-T cell therapy showed a similar efficacy and safety in younger and older patients (>70 years). Consequently, this latter group should receive CAR-T cell therapy if treatment criteria are met.
Disclosures
Bailen:Kite-Gilead: Honoraria, Other: travel; Pfizer: Other: travel; Jazz Pharmaceuticals: Research Funding. Kwon:Jazz: Speakers Bureau; Pfizer: Speakers Bureau; Kite-Gilead: Consultancy, Speakers Bureau. Iacoboni:AstraZeneca: Honoraria; MSD: Honoraria; Gilead Sciences: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Honoraria; Autolus: Consultancy; Miltenyi: Consultancy, Honoraria; Abbvie: Honoraria. Reguera:BMS: Speakers Bureau; AMGEN: Speakers Bureau; KITE: Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Lopez Corral:Gilead Sciences: Honoraria, Other: travel support; Janssen: Honoraria, Other: travel support; Novartis: Honoraria, Other: travel support. Ortiz-Maldonado:Kite: Consultancy, Honoraria; Pfizer: Consultancy; Miltenyi Biomedicine: Consultancy; Celgene BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy. Guerreiro:Novartis: Honoraria, Other: travel; Kite-Gilead: Honoraria, Other: travel; BMS: Honoraria, Other: travel support; MSD: Honoraria, Other: TRAVEL; Pierre Fabre: Honoraria, Other: travel. Bastos-Oreiro:Kite-Gilead: Honoraria, Other: travel. Carpio:BMS: Consultancy; Novartis: Honoraria; Regeneron Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria. Ribera:Pfizer: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Takeda: Consultancy; AMGEN: Research Funding; Novartis: Consultancy; Incyte: Consultancy, Research Funding. Sureda Balari:Takeda: Consultancy, Speakers Bureau; Kite: Consultancy, Speakers Bureau. Hernandez Boluda:Pfizer, BMS, Incyte, and Novartis: Membership on an entity's Board of Directors or advisory committees. Martinez-Cibrian:Kite: Honoraria, Other: Travel support. Martin Garcia-Sancho:F. Hoffmann-La Roche Ltd, BMS/Celgene, Janssen, Gilead/Kite, Takeda, Eusa Pharma, Abbvie: Honoraria; F. Hoffmann-La Roche Ltd, BMS / Celgene, Kyowa Kirin, Novartis, Gilead / Kite, Incyte, Lilly, ADC Therapeutics America, Miltenyi, Ideogen, Abbvie, Sobi: Consultancy; AbbVie: Consultancy, Honoraria; Ideogen: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; ADC Therapeutics America: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Gilead / Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Eusa Pharma: Consultancy, Honoraria; Kyowa Kirin: Consultancy, Honoraria; Clinigen: Consultancy; Roche: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Barba:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal